Carbohydrates
- Carbohydrates: molecules made of carbon, hydrogen and oxygen(rone carbon+one H20 ratio)
- carbohydrate chains:
- monosaccharides
- disaccharides
- polysaccharides
Monosaccharides
- the position of the carbonyl(C=O) group categorize the sugars:
- glucose has an aldehyde(H-C=O) group, it is aldose
- fructose has the carbonyl group with its #2 C, it is ketose(forms a ketone group)
- Glucose and its isomers
- monosaccharide: glucose, galactose, fructose have the same chemical formula(C6H12O6)
- isomers: differ in the organization of their atoms
- glucose and galactose=stereoisomers
Ring forms of sugars
- glucose's main configuration: 6-membered ring(pyranose)
- fructose's main configuration: 5-membered ring(furanose)
- glucose's alpha form and beta form
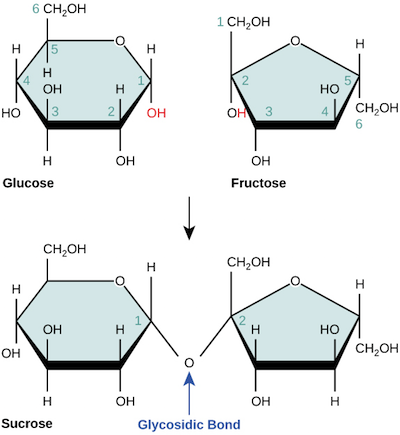
Disaccharides and dehydration reaction
- Dehydration reaction=condensation reaction, dehydration synthesis <-> Hydrolysis
- the hydroxyl(OH) group+H of another > releasing H2O and forming a glycosidic linkage(kind of covalent bond)
- glucose-O-fructose=sucrose highlight is a glycosidic linkage
- 1-2 glycosidic linkage=1 carbon of glucose-O-2 carbon of fructose
- disaccharides: lactose(glucose+galactosee), maltose(glucose+glucose), sucrose(glucose+fructose)
- Polysaccharides: starch, glycogen, cellulose, chitin..
Storage polysaccharides
- starch: the stored form of sugars in plants
- a mixture of amyose and amylopectin *alpha form
- Amylose: unbranched chains of glucose monomers connected by 1-4 linkages
- Amylopectin: branched chains of glucose monomers, most is 1-4 but 1-6 occur periodically
- *cellulose is made of glucose monomers in their beta form
- *chitin resembles cellulose
Lipid
Triglycerides
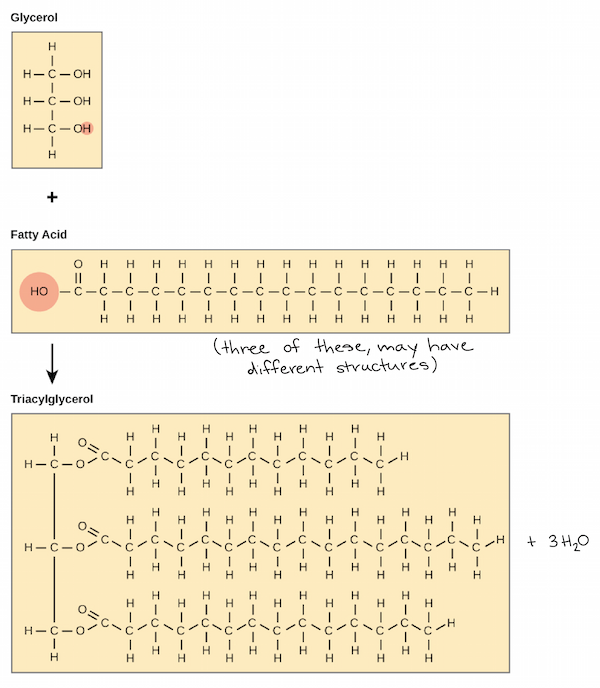
- triglycerides(in blood)=fat=trayacylglycerol: a glycerol backbone+3 fatty acid tails
- glycerol: an organic molecule with 3 hydroxyl(OH) groups
- fatty acid: a long hydrocarbon chain attached to a carboxyl(C=O-OH) group
- the hydroxyl groups(on the glycerol backbone)-"dehydration synthesis reaction"-the carboxyl group(of fatty acids)
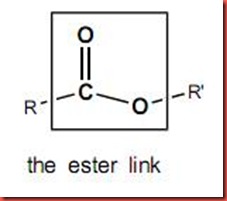
- it yields 3 fatty acid bound to glycerol via ester linkage
- *acyl group is a kind of carbonyl group
- *as a whole, fatty acid is not soluble despite it has a polar head but also has a longer non-polar carbon chain
Saturated and unsaturated fatty acids
- saturated: only single bonds between neighboring C in the hydrocarbon chain
- solid at room temperature, dense like butter
- unsaturated: hydrocarbon has a double bond(monounsaturated) or double bonds(polyunsaturated)
- liquid form at room temperature, less dense like oil
- cis configuration: 2 H on the same side, makes bent
- trans configuration: 2 H on the opposite side, makes no bent, bad for health
Other lipids
- (bees)waxs, phospholipids, steroids, cholesterol(the most common steroids)...
- phospholipids: major components of the membrane
- has a backbone of glycerol and 2 fatty acid tails
- 3rd C of the glycerol backbone is occupied by a phosphate group
- amphipathic molecule means: has a hydrophobic part and hydrophilic part
Proteins
Amino acids
- the building block of proteins
- consists of: alpha carbon, amino group(NH2), carboxyl group(COOH) and H
- in physiological pH condition:
- amino group is protonated(grab H), bears a positive charge
- carboxyl group is deprotonated(throw H), bears a negative charge
- R group defines amino acids
Peptide bonds
- carboxyl group(of the amino acid) reacts with the amino group(of an incoming amino acid)+releasing a water molecule
- directionality: amino terminus(N-terminus)on the left and carboxyl terminus(C-terminus) on the right
Protein structure
- Primary structure: the sequence of amino acids in a polypeptide chain
- Secondary structure: local folded structures within a polypeptide, due to interactions between atoms of the backbone
- a(alpha) helix, B(beta) pleated sheet: carbonyl O+amino H
- in an a helix: the carbonyl O is (hydrogen)bonded to amino H that is 4 down the chain(amino acid #1+amino acid #5)
- B pleated sheet has parallel and antiparallel form
- Tertiary structure: 3D structure of a polypeptide, due to interactions between the R groups(side chains)
- R group interactions: the whole series of non-covalent bonds
- Disulfide bonds: one special type of covalent bond in tertiary structure, between the sulfur-containing side chains of cysteines
- Quaternary structure: for some proteins made up of multiple polypeptide chains(subunits)
- same interactions that contribute to tertiary structures hold the subunits, form quaternary structure
Nucleic acids
- nucleic acids made of nucleotides
- 2 varieties: deoxyribonucleic acid(DNA), ribonucleic acid(RNA)
- in eukaryotes DNA found in the nucleus(in membrane-bound vault)
- and DNA broken up into chromosomes
- in prokaryotes DNA found in the nucleoid(not in membrane-bound container)
- central dogma: DNA-RNA copy(messenger RNA, mRNA)-ribosomes(molecular machine to build proteins)
Nucleotides
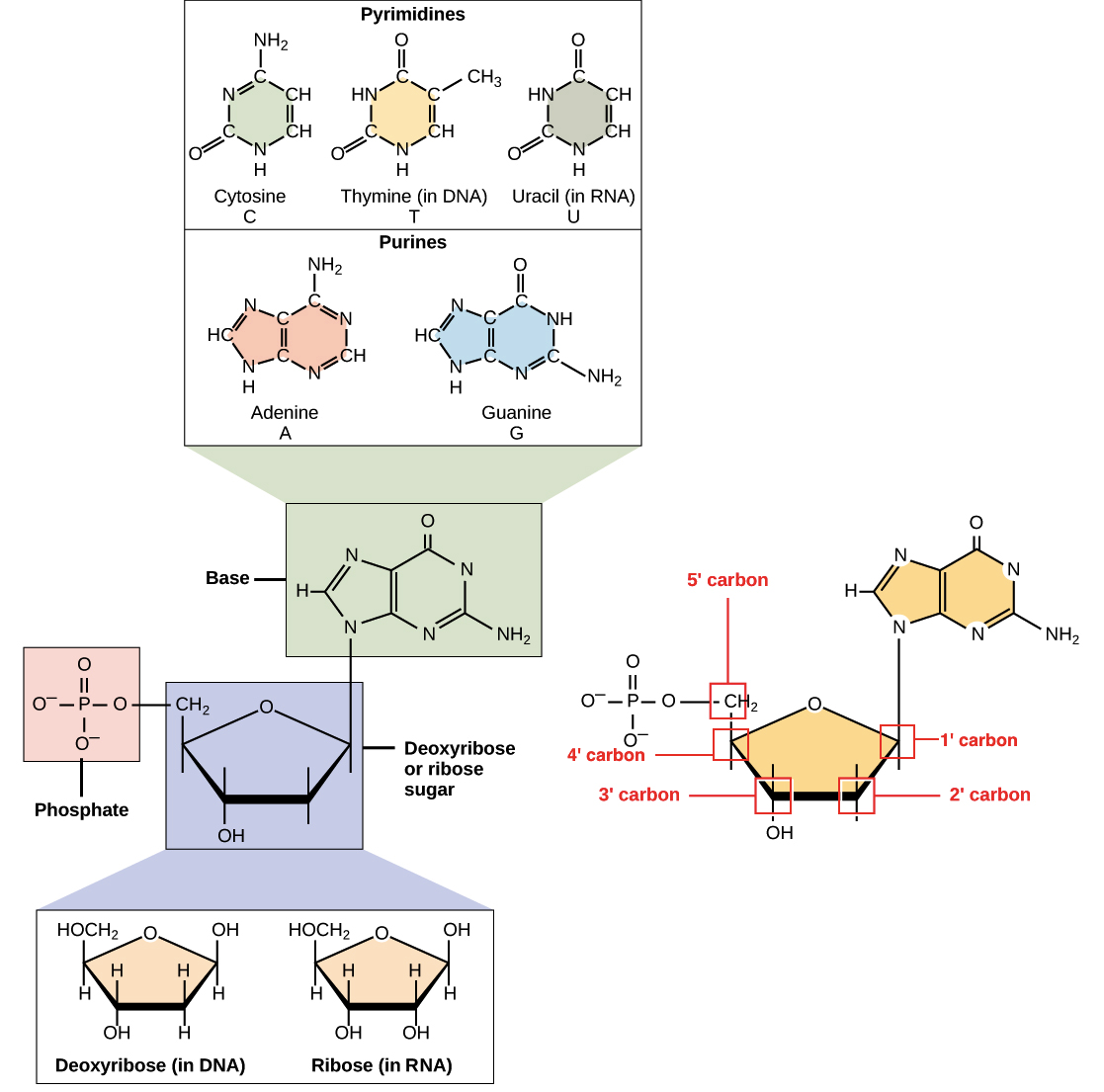
- Nucleotides made up of: nitrogenous base, five-carbon sugar, at least one phosphate group
- nitrogenous bases: adenine(A), guanine(G), cytosine(C), thymine(T)
- A, G is purines and C, T(U in RNA) is pyrimidines
- purines must pair with pyrimidines, A-T and G-C
- the five-carbon sugar in DNA is deoxyribose, in RNA, ribose
- polynucleotide chains by phosphodiester linkage: 5' to 3' direction and 3' to 5' direction(antiparallel)
| sugar-phosphate backbone |
- mRNA: intermediate between a protein coding gene&protein product
- rRNA: ribosomal RNA, a major components of ribosomes
- tRNA: transfer RNA, carry amino acids to the ribosome








No comments:
Post a Comment