Here is a fun article about making 'glowing sushi' with a glowing protein, called GFP.
article
ok, then what about the glowing beer? Is it possible to brew the glowing beer with the yeast where GFP can be activated? According to the article, the answer is no. Because the glowing protein actually is dependent on pH conditions, and a majority of target pH of finish products is below pH 3~4 where there is no way to activate for GFP.
Then, why does pH condition go down during beer fermentation? I googled.
1.
(from
there)
Fermentation is the oxidation of organic carbohydrates(
means starch) under anaerobic(
means no oxygen) conditions. The classic example is the fermentation of sugars into ethanol and carbon dioxide utilizing yeast for beer and wine production. During this process, CO2 is released into the air in the form of gas bubbles. But much CO2 is also dissolved in the water solution. CO2 dissolved in water produces carbonic acid (H2CO3) in small quantities according to the equation below:
H2O + CO2 --> H2CO3
Carbonic acid is a weak acid that will lower the pH slightly. So this is why the pH changes slightly during fermentation.
2.
(from
there)
Substantial factor:
- Organic acid excreation(means separated from a bigger body)
- Absorption of basic amino acid(basic blocks that comprise proteins, from malted grains*)
Less extent factor:
- Solution of carbon dioxide
- Absorption of primary phosphate(PO3-4)
* leave it for smarter future me...
What is free amino nitrogen(FAN)
link
3.
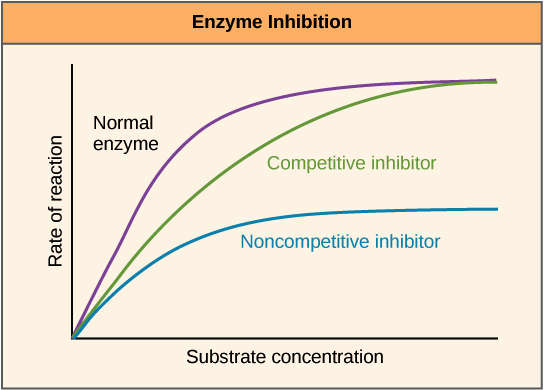
- pH affects the shape of proteins.
- (If pH is increased, this affects the shape of proteins, by disrupting bonds in the protein.)
- Enzymes are responsible for the metabolic processes that occur.
- Enzymes are proteins.
- Enzymes work best in acidic condition, when pH is lower, which bend the protein into the correct shape to allow fermentation to occur.
conclusion: lower pH is good for your wort.
4.
and the good basic lesson about pH for homebrewers is
here
leave it for the future me, too.
Yeast cells take in ammonium ions (which are strongly basic, similar to alkaline) and excrete organic acids (including lactic acid)