Overview of metabolism
- breaking down glucose=cellular respiration
- C6H126O6+6O2 -> 6CO2+6H20+energy
- which energy captured by the ATP(adenosine triphosphate)
- which is the energy currency of the cell
- building up glucose=photosynthesis
- anabolic(building up) needs energy
- catabolic(breaking down) releases energy
Laws of thermodynamics
- types of energy: kinetic/thermal(constant moving of atoms or molecules)/potential/chemical...
- types of system: open(energy, matter exchange)/closed(only energy)/isolated(both not exchange) system
- The first law of thermodynamics: energy cannot be created or destroyed, it can only change or be transferred
- The second law of thermodynamics: every energy transfer will increase the entropy of the universe(reduce the amount of usable energy)
- the system will tend to move towards more disordered configuration bc it's statistically much more likely
- system hates temperature-separated(organized) configuration!
Free energy
- Gibbs free energy change equation: △G=△H-T△S
- G means the change in free energy of a system(from initial to final)
- △G<0 a reaction proceed spontaneously(without adding energy) △G>0 need an input of energy
- H means the enthalpy(energy stored in bonds) change.
- △H<0 release heat, △H>0 absorb heat(from reactant to product)
- T means temperature
- S means the entropy change of the system
- △S<0 become ordered △S>0 become disordered(have more potential states)
- Endergonic reactions: require an input of energy(△G>0), non-spontaneous
- Exergonic reactions: release free energy(△G<0), spontaneous reactions
- ADP(adenosine diphosphate)+Pi+△G(=+7.3kcal/mol) -> ATP+H20
- the energy needed to make ATP is provided, for example, glucose
ATP and reaction coupling
- ATP(Adenosine triphosphate) is unstable due to negative charges in its phosphate tail
- phosphoanhydride bonds: bonds between the phosphate groups, high-energy
- ATP+H20 <-> ADP+Pi+energy
- △G for the hydrolysis of 1 ATP into ADP(+Pi)=-7.3kcal/mol(=-30.5kJ/mol) @standard conditions
- -14kal/mol(=-57kJ/mol) @in a living cell
- ATP in reaction coupling: the formation of sucrose
- the first reaction: a phosphate group is transferred from ATP to glucose, forming "glucose-P(glucose 6 phosphate)"
- Hexokinase: provides ions(Mg), make electrons(O- in phosphate tail) busy
- the second reaction: glucose-P intermediate reacts with fructose to form sucrose
Introduction to enzymes
- transition state: to break the bonds, molecules must be bent, unstable state, at higher energy level than both reactants and products
- activation energy(EA): initial energy input to proceed the reaction(independent to whether it's endergonic, exergonic)
- catalyst=enzymes: lower the activation energy, increase reaction rate
- (enzyme's)substrate
- active site
- environmental effects on enzyme: temperature, pH(if both extreme, enzyme denature)
- induced fit
Enzyme regulation
- enzyme cofactor: non-protein part of an enzyme, help the enzyme do its function
- organic cofactor=coenzyme like NAD(NAD+H-->NADH)
- regulatory molecules: molecules regulate enzyme. activator(encourage), inhibitor(discourage)
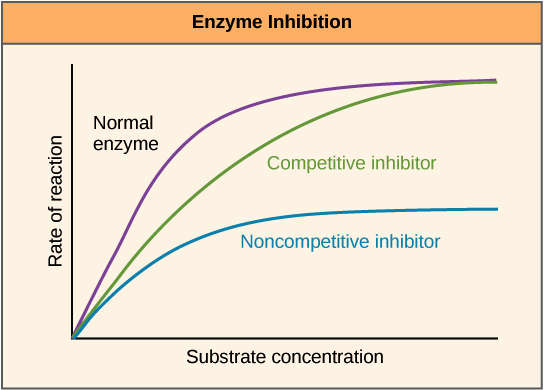
- competitive inhibition: substrate and inhibitor compete for the enzyme(active site). one attached, another can't.
- allosteric competitive inhibition: inhibitor attach to a not-active site, which prevents substrate from attaching the active site
- noncompetitive inhibition: both can attach to an enzyme, but the activity doesn't happen.
- V0(initial velocity): the amount of product/unit at the start of reaction
- Vmax(maximum velocity): the maximum rate of reaction, depends on enzyme concentration
- Vmax/2=Km: how quickly reaction rate increases with substrate concentration, altered by inhibitors
- with a competitive inhibitor: Vmax is unchanged(with more substrates, can reach the normal Vmax), Km is higher
- with a noncompetitive inhibitor: Vmax is lower than normal Vmax, Km is unchanged(bc inhibitors lower the number of (usable)enzyme)
- Michaelis-Menten equation






No comments:
Post a Comment