Introduction to the atom
- 1 Mole=6.02*1023 = Avogadro's number
- 1 Mole(6.02*1023) of 12C atoms in 12g of C
- mole's role: switching between amu and g
- 1g=1mole of amu=6.02*1023amu
- 1more of 27AI=27grams
- 1mole of 56Fe=56grams
- A(mass number)=Z(atomic number=proton number)+N(neutron number)
- 18Fe- consists of: 9 proton, 9 neutron, 10 electron
ions and compounds
- in covalent bonds, atoms share electrons
- a group of atoms joined by covalent bonds is a molecule
- there is NO such thing as a molecule of an ionic bond
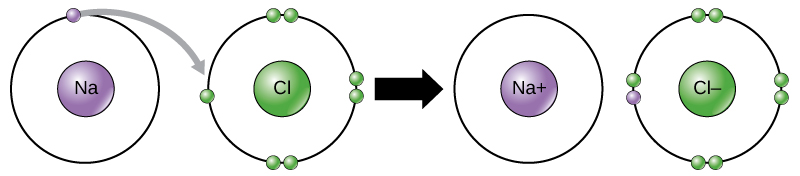
- in ion bonds, electrons are fully transferred between two atoms, so that ions are formed
- ionic bond: two oppositely charged ions attract each other
- representing molecules: chemical formula, structural formula
- *the group 3A elements link
names and formulas of ionic compounds
- cation: ion with net positive charge
- anion: ion with net negative charge
- predicting charges on monatomic cations and anions

- groups 1,2,13,14: usually give away valence electrons to become ions(C is sometimes an exception)
- groups 15,16,17: usually negative charge, because is more likely to gain electrons not lose
- most of the transition metals(d-block)are polyvalent
- naming of transition metal cation: chromium(II)chloride=Cr2+
- naming anions: add suffix '-ide'
- formulas and naming of basic ionic compounds:
- always name the cation(+)before the anion(-)
- when naming the cation, don't include the word ion or charge unless it's a polyvalent ion
- any ionic compounds have a net charge of zero(they cancle each other)
- polyatomic ions: the ion that contains more than one atom
- polyatomic ion is formed when a neutral molecule(covalent bond) gain or lose electrons
- the chemical formula of calcium hydroxide is: Ca(OH)2 not CaOH2
Isotopes and mass spectrometry
- isotope: same # of proton, electron but different # of neutron
- atomic weights: weighted average calculated by summing up each isotope's atomic mass
- mass number=number of netrons+number of protons(atomic number)
- 1u(amu)=1/12 of the mass of single neutral atom of carbon-12 *12 is the mass #
- mass of a neutron=1amu
- mass of a proton=1amu
- mass of an electron=considered to be zero amu
- atomic mass=how many protons and neutrons*1amu(not exact, but close)



No comments:
Post a Comment