Introduction to cellular respiration
- adenosine triphosphate(ATP)
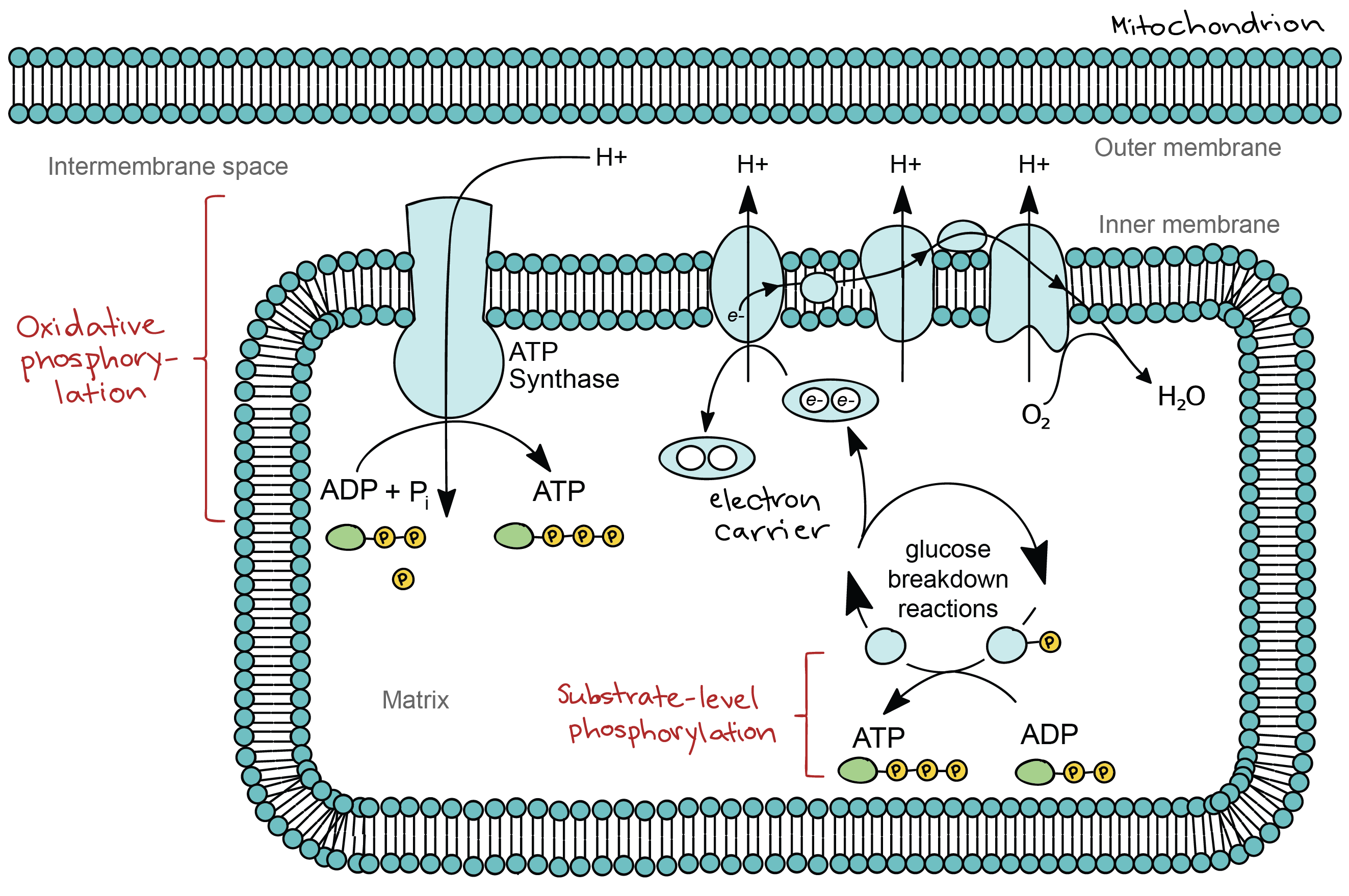
- substrate-level phosphorylation
- oxidative phosphorylation
- cellular respiration
- Electron carriers(shuttles): small organic molecules which pick up electrons from one molecule and drop them off with another
- 2 types of electron carriers in cellular respiration: NAD+(nicotinamide adenine dinucleotide)/ FAD(flavin adenine dinucleotide)
- Redox reactions(oxidation-deduction reactions): reactions involving electron transfers
- one molecule loses electrons: oxidized(OIL)
- one molecule gains electrons: reduced(RIG)
- in the context of biology:
- if a carbon-containing molecule gains H or loses O, it's likely been reduced(gained electrons or electron density)
- if a carbon-containing molecule loses H or gains O, it's likely been oxidized(lost electrons or electron density)
- The atoms that H is usually bound to(C, O, N, P)are more electronegative than H
- so, if a H and its electron join a molecule, the rest is going to hog the electron(become reduced)
- O is more electronegative than any of the other major atoms
- if it joins a molecule, it's likely going to pull away electron density(oxidize)
- electrons are at a higher energy level when they're with less electronegative atoms(C or H)
- electrons are at a lower energy level when they're with more electronegative atoms(O)
- in a breakdown of glucose, electrons are moving from higher energy level to lower(energy is releasing and captured!)
Steps of cellular respiration
- Glycolysis: glucose converted into 2 pyruvates, ATP made, NAD+ is converted to NADH
- *glycolysis without oxygen=fermentation. other 3 stages require oxygen to occur
- Pyruvate oxidation: pyruvate converted into acetyl CoA, CO2 released, NADH generated
- Citric acid cycle: end product of the cycle+acetyl CoA=initial product of the cycle. ATP, NADH, FADH2 produced, CO2 released
- Oxidative phosphorylation: electrons move down the chain, energy released, to pump protons out of matrix, forming a gradient, protons flow back into the matrix through an ATP synthase, making ATP
Glycolysis
Highlights of glycolysis
- takes place in the cytosol of a cell
- catalyzed by enzyme, phosphofructokinase
- energy requiring phase
- glucose molecule rearranged
- two phosphate groups(spend 2 ATP) are attached to it
- make the modified sugar: fructose-1,6-bisphosphate
- break into: glyceraldehyde-3-phosphate and DHAP(soon converted to the former one)
- energy releasing phase
- glyceraldehyde-3-phosphate converted into pyruvate*2=2 pyruvates
- 2 ATP and 1 NADH made *take place twice=4 ATP and 2 NADH
Detailed steps: Energy requiring phase
- a phosphate group is transferred from ATP to glucose, making glucose-6-phosphate
- glucose-6-phosphate is converted into fructose-6-phosphate
- a phosphate group is transferred from ATP to fructose-6-phosphate, making fructose-1,6-bisphosphate *phosphofructokinase*
- fructose-1,6-bisphosphate splits into glyceraldehyde-3-phosphate and DHAP
- DHAP is converted into glyceraldehyde-3-phosphate
Detailed steps: Energy releasing phase
- glyceraldehyde-3-phosphate lose 2 electrons and 2 protons, reducing NAD+->NADH and H+ *release energy* forming 1,3-biphosphoglycerate
- 1,3-biphosphoglycerate donate a phosphate group to ADP(making ATP), turning into 3-phosphoglycerate
- 3-phosphoglycerate is converted into 2-phosphoglycerate
- 2-phosphoglycerate loses 1 molecule of water, becoming phosphoenolpyruvate(PEP)
- PEP(unstable) donates a phosphate group to ADP(making ATP), turning into pyruvate
What happens to NADH
- if there's no NAD+ , glycolysis come to a halt
- when oxygen is present: through the electron transport chain, regenerating NAD+
- when oxygen is absent: NADH donates electrons to an acceptor molecule, regenerating NAD+
Pyruvate oxidation
Overview of pyruvate oxidation
- in eukaryotes, it takes place in the mitochondrial matrix
- in prokaryotes, it happens in the cytoplasm
- (glucose->)2 pyruvate converted into 2 acetyl CoA, producing 2 NADH and 2 CO2
- acetyl CoA acts as fuel for the citric acid cycle
Pyruvate oxidation steps
- a carboxyl group is removed, released as a CO2
- via step1, an acetyl group is oxidized, lose electrons, NAD+->NADH
- an acetyl group is attached to Coenzyme A(CoA) to form acetyl CoA
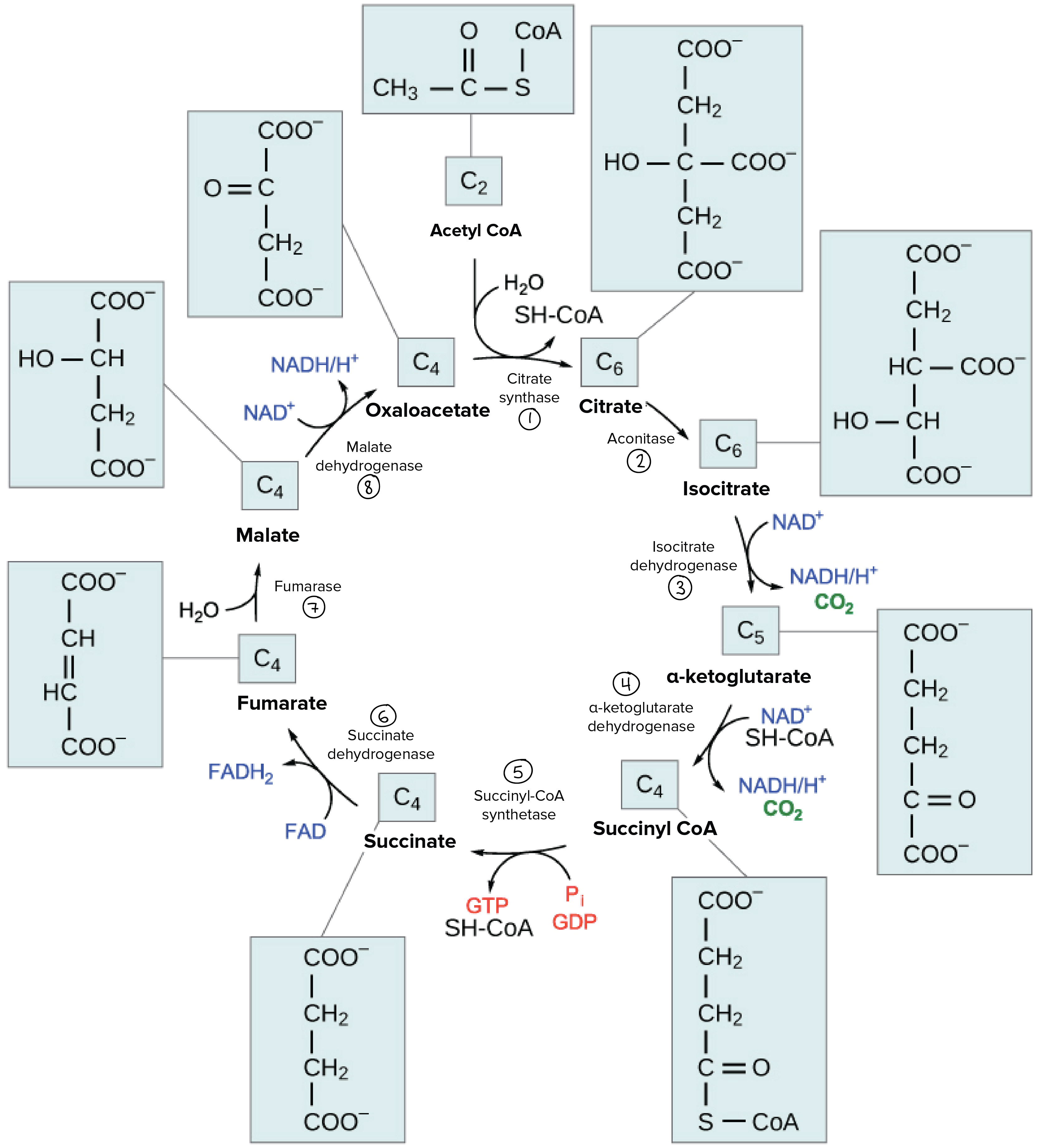
The citric acid cycle
Overview of the citric acid cycle
- in eukaryotes, it takes place in the mitochondrial matrix
- in prokaryotes, it happens in the cytoplasm
- a closed loop, 8 major steps
- one turn of the citric acid cycle=2 CO2, 3 NADH, 1 FADH2, 1 ATP or 1 GTP
- the cycle goes around twice/glucose
Steps of the citric acid cycle
- acetyl CoA+oxaloacetate=CoA group+citrate
- citrate->isocitrate
- isocitrate is oxidized->a-ketoglutarate+releasing 1 CO2, NAD+->NADH *isocitrate dehydrogenase
- a-ketoglutarate is oxidized->succinyl CoA+releasing 1 CO2, NAD+->NADH *a-ketoglutarate dehydrogenase
- succinyl CoA->succinate, ADP/GDP is forming ATP/GTP
- succinate is oxidized->fumarate, FAD->FADH2
- fumarate+water->malate
- malate is oxidized->oxaloacetate, NAD+->NADH
Oxidative phosphorylation
Overview: oxidative phosphorylation
- found in the inner membrane of the mitochondria
- electron transport chain+chemiosmosis=oxidative phosphorylation
- delivery of electrons by NADH and FADH2
- electron transfer and proton pumping
- splitting of oxygen to form water
- gradient-driven synthesis of ATP
The electron transport chain
- a collection of membrane-embedded proteins and organic molecules
- in eukaryotes, it is found in the inner mitochondrial membrane
- in prokaryotes, it is found in the plasma membrane
- electrons go from a higher(less electron hungry) to a lower(more electron hungry) energy level
- regenerates electron carriers(NADH, FADH2)
- makes a proton gradient
- NADH is very good at donating electrons, it can transfer its electrons directly to complex I
- FADH2 is less good at donating electrons, it feeds electrons into the chain through complex II(doesn't pump H+)
- complex II: electron carrier 'ubiquinone(Q)', delivering electrons to complex III
- complex III: another carrier 'cytochrome C(cyt C)', delivering electrons to complex IV
- complex IV: passes the electrons to O2
Chemiosmosis
- proton gradient=electrochemical gradient=proton-motive force
- ATP synthase: adding phosphate to ADP, capturing energy(from the H+ gradient) as ATP
- energy stored in the proton gradient but not used to synthesize ATP->HEAT
Variations on cellular respiration
Fermentation and anaerobic respiration
- when no oxygen as an acceptor at the end of the electron transport chain
- fermentation pathways(glycolysis+extra reactions) come to join
- extra reactions: make alcohol(yeast), lactic acid(muscle)
- anaerobic cellular respiration: some bacteria or archaea use sulfate as an acceptor
Fermentation
- glycolysis+extra reactions
- the pyruvate made in glycolysis->NO oxidation, NO citric acid cycle->NO electron transport chain run
- NADH made in glycolysis cannot turn back into NAD+
- extra reactions: is to regenerate NAD+
Alcohol fermentation
- NADH donates its electrons to a derivative of pyruvate, producing ethanol
- a carboxyl group is removed from pyruvate, releasing CO2, producing acetaldehyde
- NADH passes electrons to acetaldehyde, regenerating NAD+, forming ethanol
Lactic acid fermentation
- NADH transfers electrons directly to pyruvate, generating lactate(deprotonated form of lactic acid)
- bacteria that make yogurt
- muscle cells that have too little oxygen(when exercising very hard)
Facultative and obligate anaerobes
- facultative anaerobes: can switch between aerobic/anaerobic pathways(=fermentation)
- obligate anaerobes: can live only in the absence of oxygen







No comments:
Post a Comment